- +91-9845055322
- [email protected]
- Mon - Fri: 9:00 - 18:30
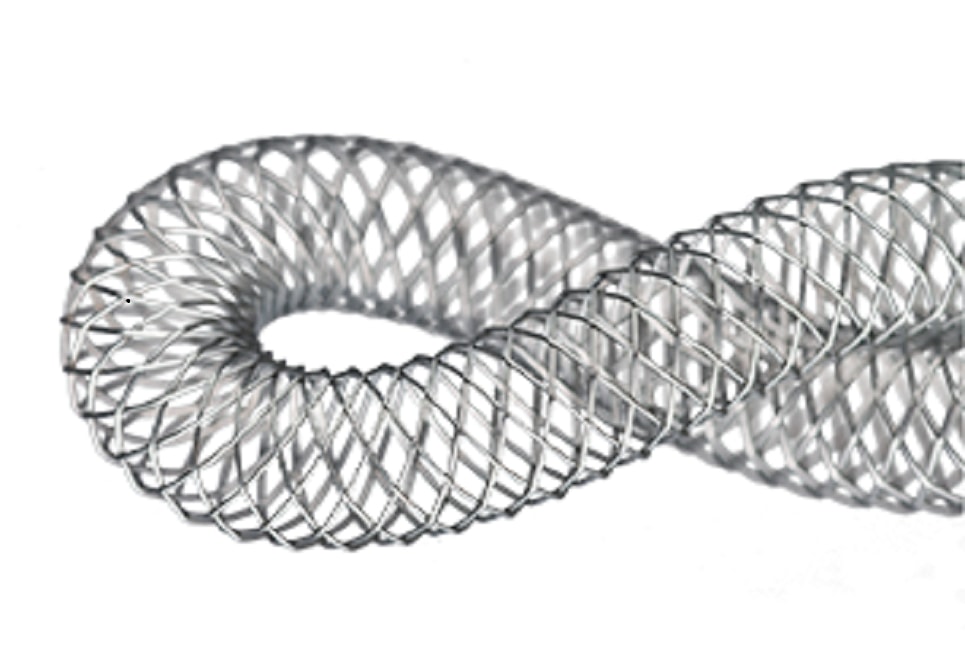
The Supera Peripheral Stent System is a unique class of technology that allows the Supera stent to mimic the anatomy’s natural movement and is an effective option for the dynamic environment of the SFA and popliteal arteries. Supera is clinically proven, demonstrating excellent patency in the SUPERB trial and has been analyzed in over 1,600 patients worldwide in the SUPERB trial and 13 retrospective studies
The Supera Peripheral Stent System is a unique class of technology that allows the Supera stent to mimic the anatomy’s natural movement and is an effective option for the dynamic environment of the SFA and popliteal arteries. Supera is clinically proven, demonstrating excellent patency in the SUPERB trial and has been analyzed in over 1,600 patients worldwide in the SUPERB trial and 13 retrospective studies.
Interwoven Stent Design
The Supera stent mimics the natural structure and movement of the anatomy. The innovative, interwoven nitinol design creates a stent that supports rather than resists the vessel.2 By pre-dilating and matching the stent and vessel 1:1, Supera stent supports the vessel with minimal chronic outward force.5,6
Strength & Flexibility
Supera stent has unparalleled strength and flexibility, with more than four times the compression resistance of all other standard nitinol stents.3,7 In severely calcified lesions, Supera stent has visible compression resistance, maintaining a round, open lumen for normal, healthy blood flow in challenging anatomy.
1,636 patients studied, see Werner, et al., Treatment of complex atherosclerotic femoropopliteal artery disease with a self-expanding nitinol stent: midterm results for the Leipzig SUPERA 500 registry, EuroIntervention 2014:10:861-868. (including Leipzig SFA, Leipzig Popliteal and S500 LL), 439 patients; Goverde, et al., AURORRA registry: Experience with high radial force interwoven nitinol stents in femoropopliteal arteries, LINC 2013, 117 patients; Molenaar, et al., Interwoven self-expanding nitinol stents for long complex SFA and popliteal lesions CWZ, LINC 2012, 178 patients; Goltz, et al., Endovascular Treatment of Popliteal Artery Segments P1 and P2 in Patients with Critical Limb Ischemia, J Endovasc Ther 2012;19:450-456, 40 patients; Chan, et al., Primary stenting of femoropopliteal atherosclerotic lesions using new helical interwoven nitinol stents, JVS Feb 2014:59:384-391, 78 patients; Pacanowski, et al., RESTORE: Interwoven Stents in the Real World, The Initial United States Experience with the Use of the Supera Stent in the SFA and Popliteal Artery, LINC 2013, 147 patients; George , et al., SUPERA interwoven nitinol Stent Outcomes in Above-Knee Interventions (SAKE) study, JVIR 25.6: 954-61, 80 patients; Dumantepe, et al., Treatment of complex atherosclerotic femoropopliteal artery disease with a self-expanding interwoven nitinol stent: Midterm results, Vascular February 2015, 36 patients; Varcoe, R., A Real World Experience Using SUPERA in Long, Challenging Lesions, LINC 2015, 105 patients; Brescia, et al., Stenting of femoropopliteal lesions using interwoven nitinol stents, J Vasc Surg. 2015 Mar 6, 38 patients; Mussa, et al., The SUPERA stent for superficial femoral artery lesions even with calcification, Charing Cross 2015, 41 patients; Lojedo Vicent, et al., Experiencia inicial en la colocacion de endoprotesis Supera en eltratmiento de la patologia arterial en sector femoropopliteo , XIV Congreso de la SERVEI 2015, 29 patients; Palena, et al., SUPERSUB Trial: 1-yr outcomes of Supera Subinitimal stenting in CLI patents, LINC 2016, 34 patients; Rosenfield, K., et al., Wire-Interwoven Nitinol Stent Outcome in the Superficial Femoral and Proximal Popliteal Arteries 12 Month Results of the SUPERB Trial, Circ Cardiovasc Interv. 2015, 264 patients.
Characteristically round lumens supported by Arena, F.J., Arena, F.A. Intravascular Ultrasound Evaluation of Interwoven Nitinol Stents at Implant. J Vasc Med Surg. 2013:1;116.
Strength is defined as compression resistance The compression resistance for a 5.0 x 100 mm Supera implant is 9 kg at 53% compression. This is four times the compression resistance of all other competitors. All other products compressed 53% with less than 2.25 kg applied. Data on file at Abbott Vascular
Garcia L., et al., Wire-Interwoven Nitinol Stent Outcome in the Superficial Femoral and Proximal Popliteal Arteries 12 Month Results of the SUPERB Trial, Circ Cardiovasc Interv. 2015
Supera Peripheral Stent System Instructions for Use
Measurements taken at upper limit of labeled vessel diameter mm. Data on file at Abbott Vascular
Flexibility is defined as kink resistance measured in a tube. The Supera sizes with the lowest kink resistance, as compared to 6.0 x 100 mm standard nitinol stents, are the 5.0 x 100 and 6.0 x 100 mm implants. Data on file at Abbott Vascular